|
|
| Adaptation of protein synthesis during aging |
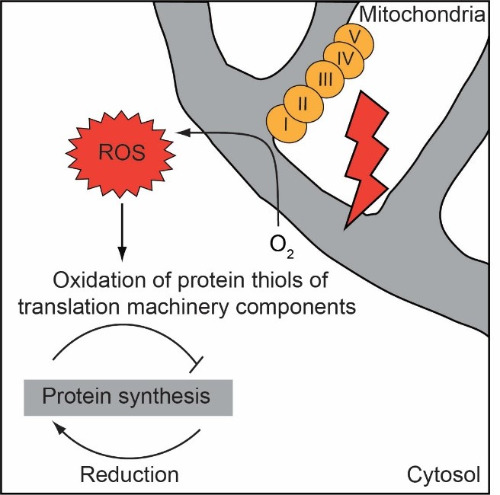
| This project aims to discover mechanisms that modulate the function of the cells during aging. Finding ways to strengthen and extend protective mechanisms of the cell could lead to expanding the organismal healthspan. Based on the recent findings we propose that one such mechanism could be the modulation of cellular protein synthesis (translation) (Wrobel*, Topf* et al., Nature, 2015). Synthesis of proteins declines during aging. However, interventions that decrease protein synthesis early in life of model organisms can be beneficial for longevity. Translation itself is regulated at multiple steps but the signal for its modulation and consequent molecular switches are little investigated. Reactive oxygen species (ROS) could be potent messengers for translation modulation because their levels increase during aging; low levels of ROS can activate thiol-based redox switches, and exogenous addition of ROS to the cell attenuates protein synthesis. We identified components of the protein synthesis machinery that can be regulated by oxidative protein modification (Topf et al., Nat Com, 2018) and study the influence of such oxidation on the translation process during cellular stress and aging. |
 |
| Non-canonical functions of molecular chaperones downstream of protein synthesis |
|
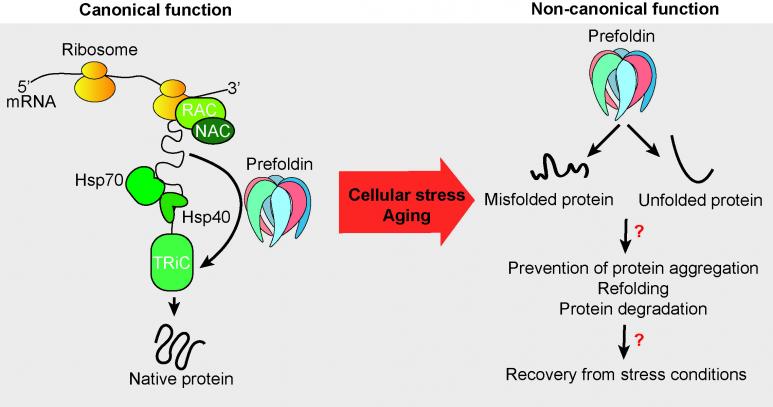
Newly synthesized polypeptides need the assistance of molecular folding chaperones and co-chaperones to obtain their correct three-dimensional structure. However, slow-down of protein production might free the chaperones to attend other cellular processes. We focus our research on the co-chaperone prefoldin which is evolutionary highly conserved. The heterohexameric complex can act in an ATP-independent manner and is predominantly known in eukaryptes to be imporantant to assist the folding of actin and tubulin. Prefoldin is involved in numerous human diseases including cancer and neurodegegneration (Tahmaz et al., 2022). Thus, discovery of non-canonical functions of prefoldin will be of pivotal importants to reveal its role within the proteostasis network to better understand human diseases that are also linked with the aging process.
|
 |
| Linking protein turnover and organismal health span |
|
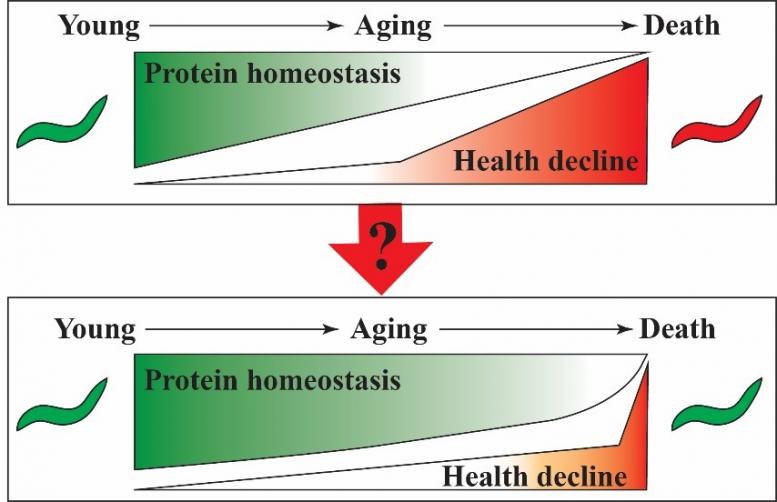
Aging is not merely a decline in cellular functions but rather a regulated process, which is evident from evolutionary conserved signaling pathways that can be genetically modulated to extend organismal lifespan. Protein homeostasis (proteostasis) collapse is a hallmark of aging and is caused by the cell's inability to manage consequences of increasing stress levels. A challenging task in aging research is monitoring the process of proteostasis decline including its key mechanisms attaining quantitative measurements. Obtaining quantifiable values that reflect the process of proteostasis decline will enable the development of interventions that can slow-down this process. To study the process of biological aging we are using the nematode C. elegans and take advantage of its tremendous genetic tractability and suitability for chemical screening approaches. Our long-term goal is the identification and characterisation of interventions that prolong protein homeostasis and can improve health deficits of aging organisms.
|
 |
|